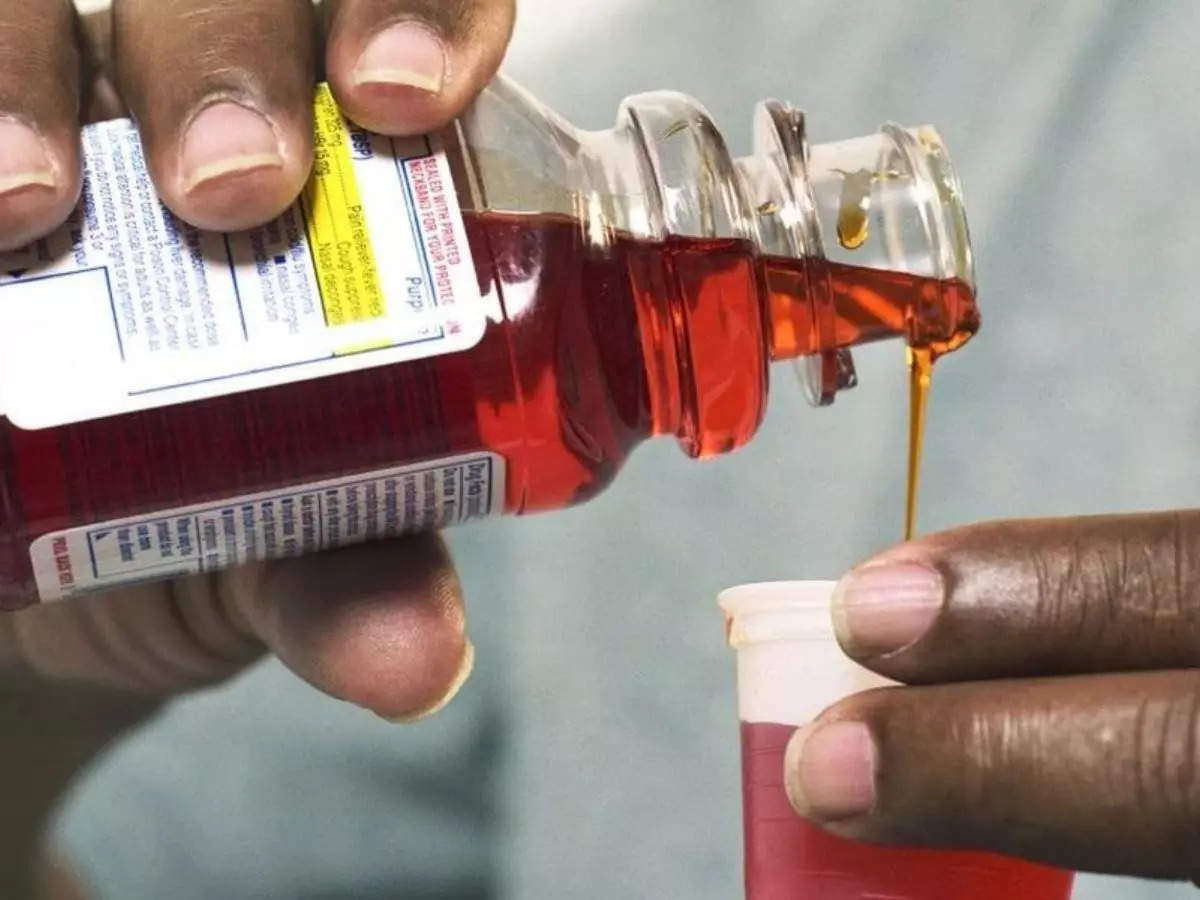
LONDON: The World Well being Group is prone to situation a wider warning about contaminated Johnson and Johnson-made kids’s cough syrup present in Nigeria final week, it stated in an e mail.
Nigeria’s regulator recalled a batch of Benylin syrup final Wednesday, having discovered a excessive stage of diethylene glycol within the product throughout routine testing.
The contaminant, alongside one other intently associated toxin, has been linked to the deaths of greater than 300 kids in Gambia, Uzbekistan, Indonesia and Cameroon since 2022, although there isn’t any proof that these incidents are linked with the brand new remembers.
The U.N. well being physique stated it places out international medical product alerts to “encourage diligence” by nationwide authorities and was possible to take action on this occasion, “topic to affirmation of sure particulars from events”.
The batch of Benylin syrup recalled was made by J&J in South Africa in Might 2021, though Kenvue now owns the model after a spin-off from J&J final 12 months.
J&J has referred requests for remark to Kenvue. Kenvue was not instantly out there for remark, however stated this week it was conducting its personal evaluation and dealing with well being authorities to find out a plan of action.
Since Nigeria’s recall, 5 different African international locations have additionally pulled the product from cabinets – Rwanda, Kenya, Tanzania, Zimbabwe and South Africa, the place the drug was made.
South Africa’s regulator has additionally recalled one other batch of the syrup, which is used to deal with coughs, hay fever and different allergic reactions in kids.
Diethylene glycol is poisonous to people when consumed and may end up in acute kidney failure, though there have been no experiences of hurt within the newest incident.
Within the 2022 circumstances, the contamination within the syrups got here from the uncooked supplies utilized by producers in India and Indonesia.
The WHO stated it was collaborating with each the producer and regulatory authority in South Africa to analyze this for the Benylin paediatric syrup, and had info on the supply of the uncooked substances used. It didn’t disclose the supply.
In response to WHO, Kenvue stated it examined the uncooked substances used within the syrup earlier than manufacture, and “no problems with concern have been famous”.
The company stated the likelihood that the syrup was counterfeit was additionally “into account as a part of investigations”.
Nigeria’s regulator recalled a batch of Benylin syrup final Wednesday, having discovered a excessive stage of diethylene glycol within the product throughout routine testing.
The contaminant, alongside one other intently associated toxin, has been linked to the deaths of greater than 300 kids in Gambia, Uzbekistan, Indonesia and Cameroon since 2022, although there isn’t any proof that these incidents are linked with the brand new remembers.
The U.N. well being physique stated it places out international medical product alerts to “encourage diligence” by nationwide authorities and was possible to take action on this occasion, “topic to affirmation of sure particulars from events”.
The batch of Benylin syrup recalled was made by J&J in South Africa in Might 2021, though Kenvue now owns the model after a spin-off from J&J final 12 months.
J&J has referred requests for remark to Kenvue. Kenvue was not instantly out there for remark, however stated this week it was conducting its personal evaluation and dealing with well being authorities to find out a plan of action.
Since Nigeria’s recall, 5 different African international locations have additionally pulled the product from cabinets – Rwanda, Kenya, Tanzania, Zimbabwe and South Africa, the place the drug was made.
South Africa’s regulator has additionally recalled one other batch of the syrup, which is used to deal with coughs, hay fever and different allergic reactions in kids.
Diethylene glycol is poisonous to people when consumed and may end up in acute kidney failure, though there have been no experiences of hurt within the newest incident.
Within the 2022 circumstances, the contamination within the syrups got here from the uncooked supplies utilized by producers in India and Indonesia.
The WHO stated it was collaborating with each the producer and regulatory authority in South Africa to analyze this for the Benylin paediatric syrup, and had info on the supply of the uncooked substances used. It didn’t disclose the supply.
In response to WHO, Kenvue stated it examined the uncooked substances used within the syrup earlier than manufacture, and “no problems with concern have been famous”.
The company stated the likelihood that the syrup was counterfeit was additionally “into account as a part of investigations”.